CDMO
Services
Interested in how our CDMO capabilities can support your needs?
We offer comprehensive solutions
At Suanfarma CDMO, we are committed to offering comprehensive solutions that allow our customers to reach all their goals to rapidly access the market and improve the lives of patients.
We are a true One-Stop Shop partner through supply chain and value chain solutions.
Supply chain

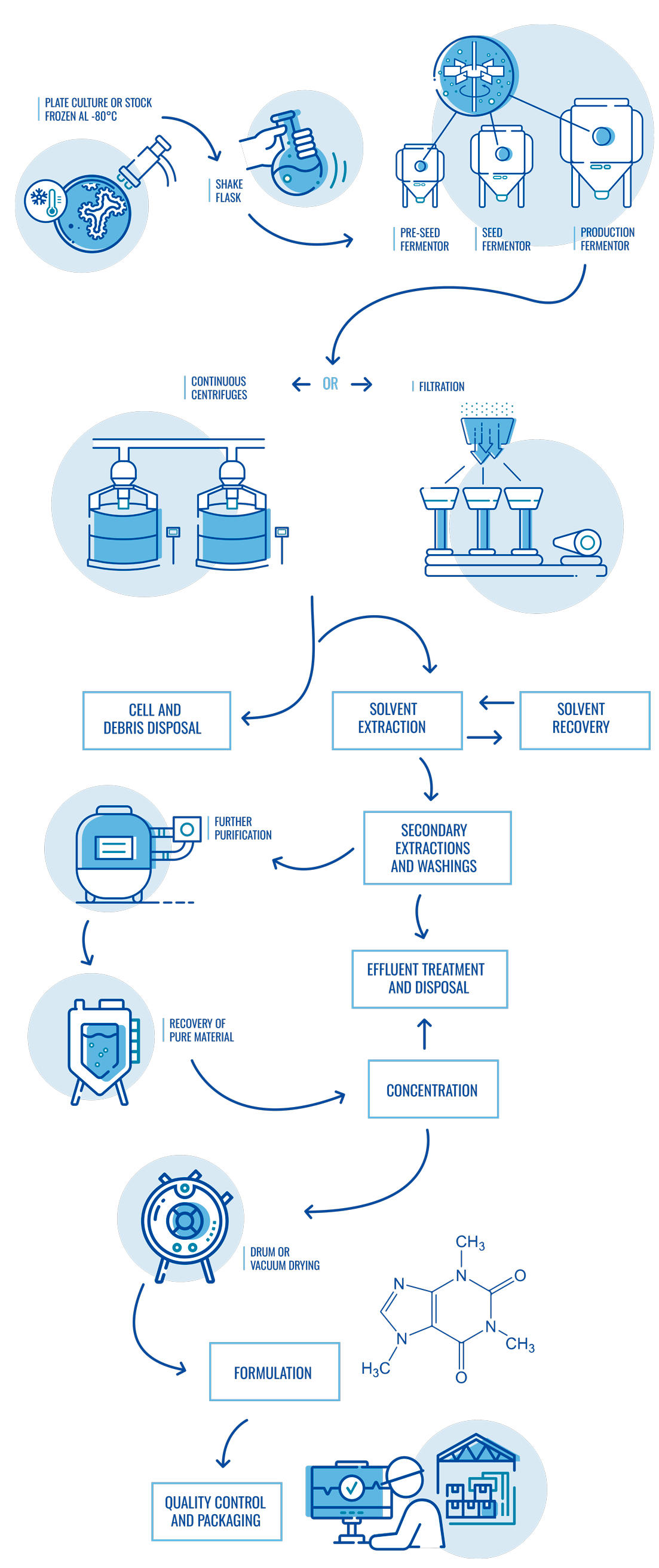
Our unique capabilities combine GMP industrial processes of fermentation, purification and chemical synthesis for a wide variety of small molecules (APIs and intermediates) and companies (Pharma, Biotech or Healthcare).
Our technologies and expertise support us to achieve success regardless of whether the molecule is innovative or generic.
As a result of our commitment to optimization, flexibility and sustainability, our clients are much more competitive in meeting market needs.
Value chain

Our services run from preclinical supply up to commercial manufacturing, continuously supporting our customers as they take molecules through development and onto the market. This is consolidated with our core values including a strong track record, scientific expertise, flexible mindset and full transparency. Our complete value chain enhances innovation in different areas. We adapt to any changes during the project, accommodate batch size requirements, and guarantee timely capacity and required business plan design.
Our goal is to offer fast and flexible CDMO services following the highest quality standards that allow our customers to reduce time to time market and secure supplies.
Our capabilities
At Suanfarma CDMO we adapt to our customers’ needs for fermentation, purification and chemical synthesis processes in small, medium and large volumes.
Our professional R&D and Industrial teams provide full support to our customers during process development and commercialization of small molecules (APIs and intermediates). Our unique manufacturing capabilities in Europe, include own laboratory, pilot plant and industrial scales which allows combining fermentation and chemical synthesis technologies in a single site.
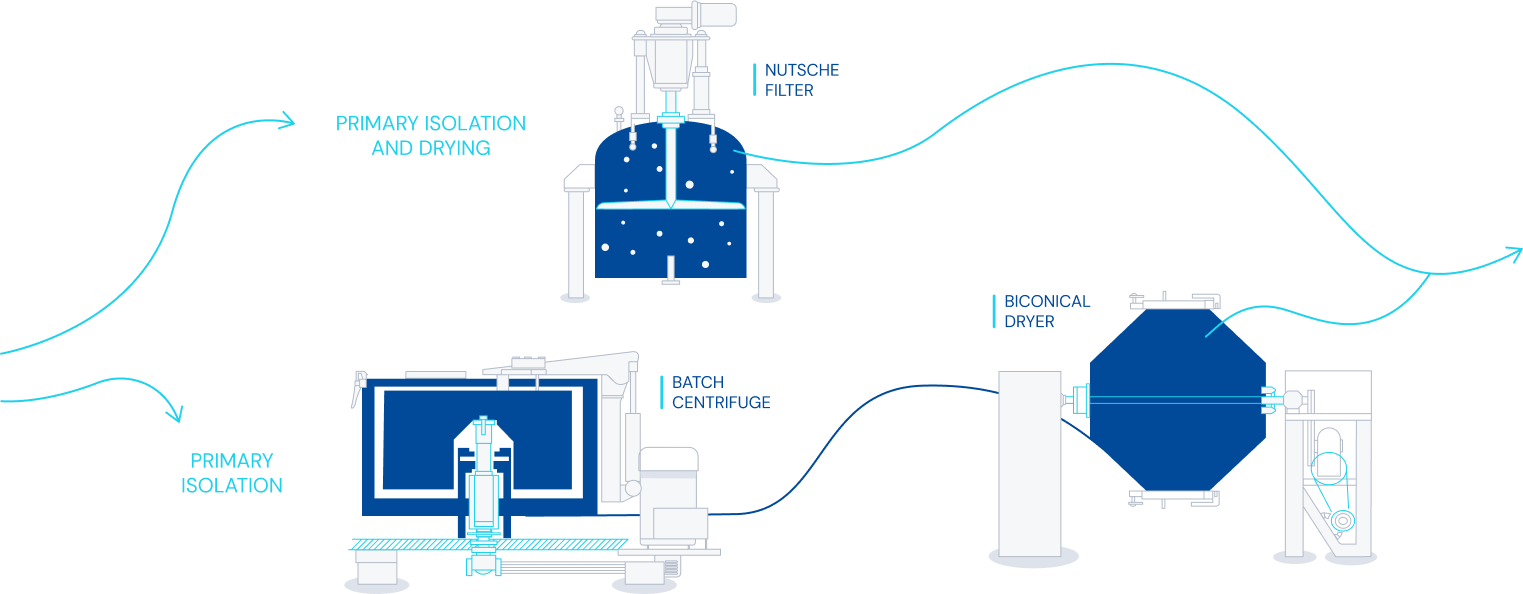
Fermentation & purification
- Small molecule fermentation and purification for human and veterinary use.
- Different types of facilities from highly automated to flexible production plants.
- Fermentation capacity with over 2.000 m3.
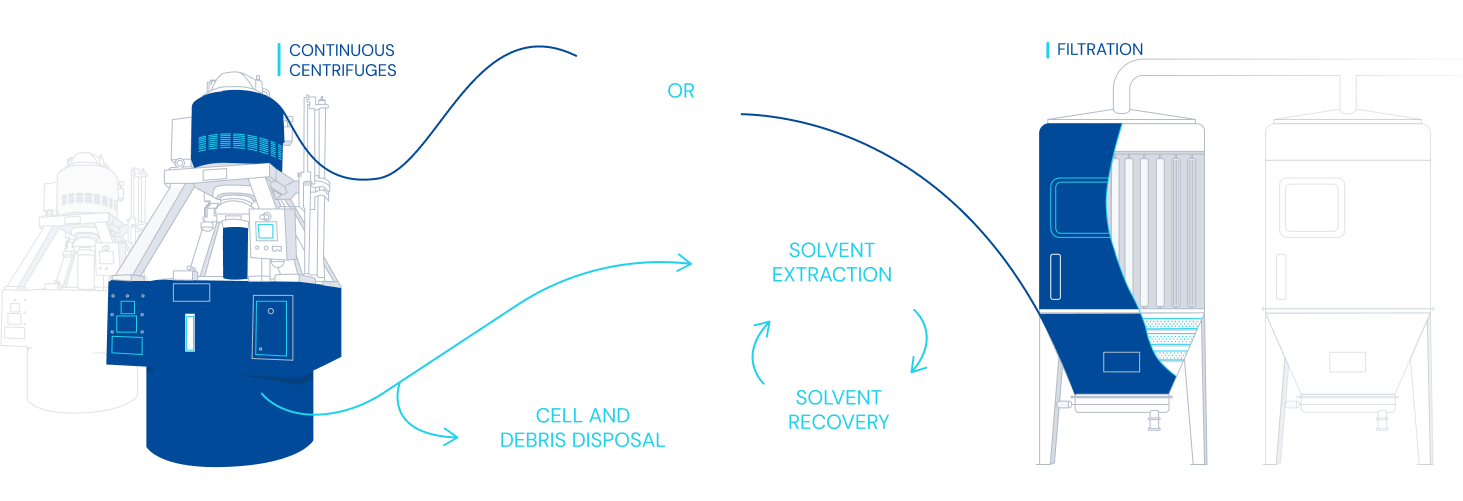
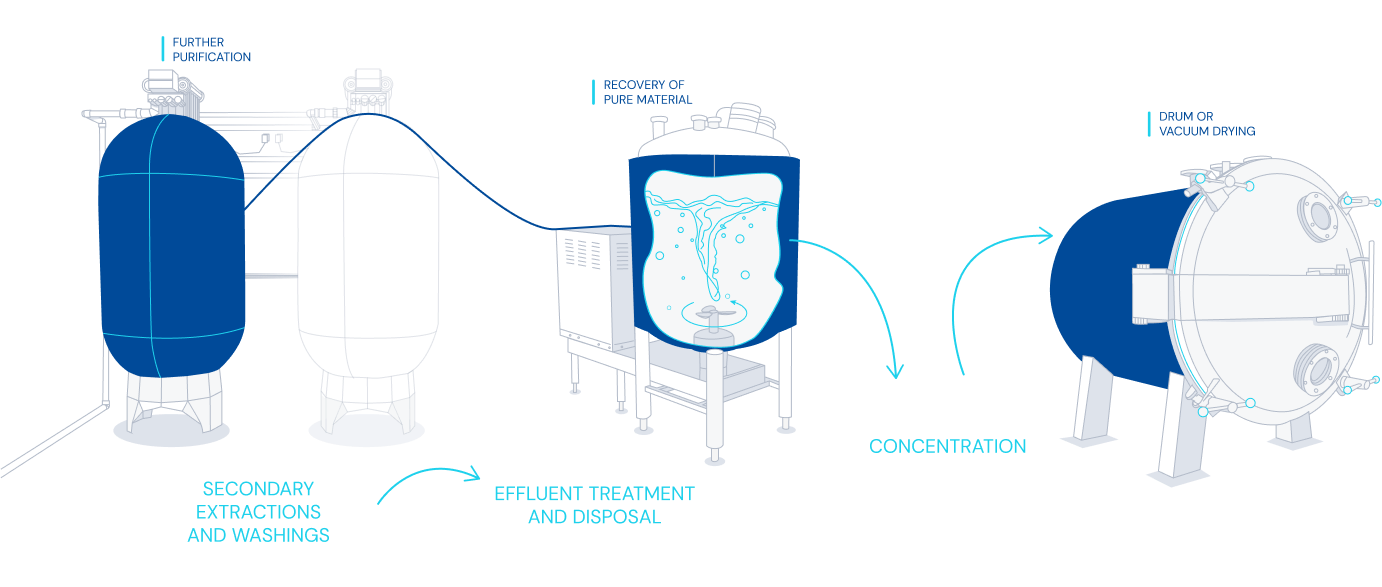
- Technologies applicable to purification including centrifugation, L-L and S-L separators, RVF, microfiltration, chromatography, filtration and dryers.
- Pilot plant with optimized design to ensure successful technology transfer and scaling-up phases.
- Expertise managing different kind of organisms for fermentative production and different types of molecules/applications.
- Technical and GMP batches.
Diagram of fermentation and purification process

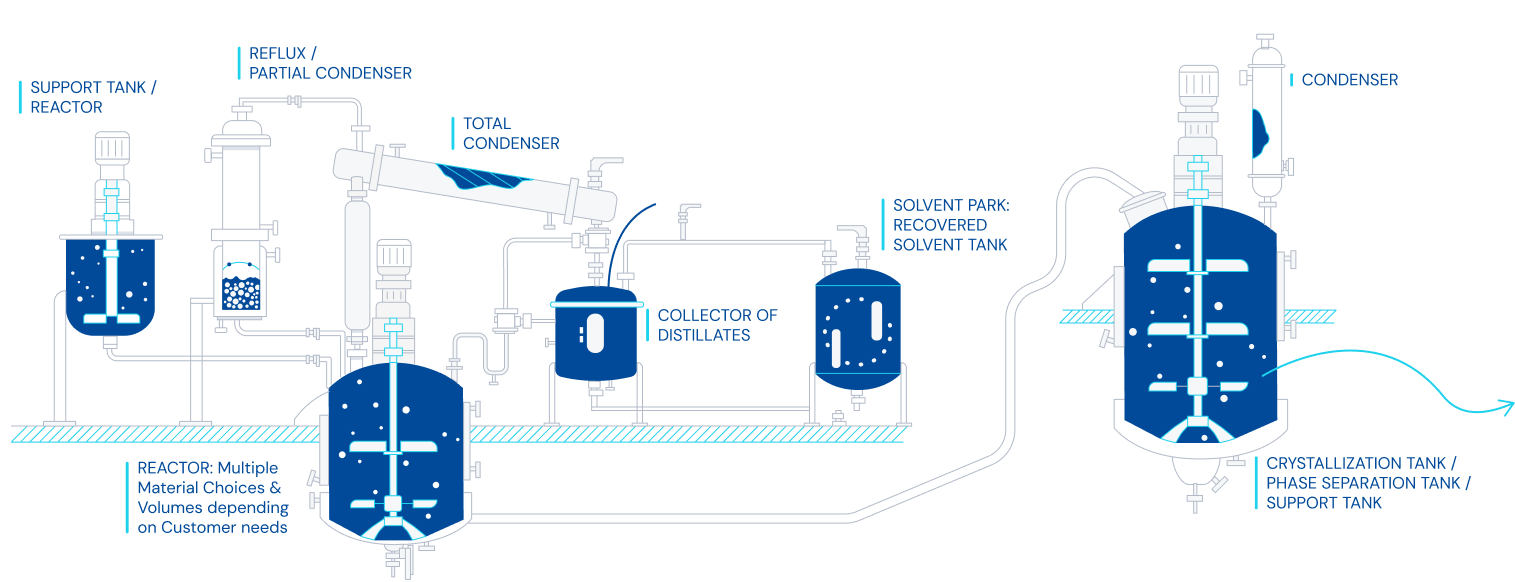
Chemical synthesis
- Chemical synthesis of small molecules for human and veterinary use.
- Different types of facilities from highly automated to flexible production plants.
- Reaction capacity with over 800 m3.
- cGMP Kilo-Lab for small scale processes.
- Expertise in complex chemical reactions, carbonylation, hydrogenation, distillation, crystallization, cryogenic reactions, and for a wide variety of drug substances.
- Stainless steel reactors, Hastelloy, special alloy and glass lined equipment, available for your projects.
- Technical and GMP batches.
Diagram of a synthesis process

Integrated platforms and policies
Innovation is key in the way we manage CDMO projects with our customers.
At Suanfarma CDMO, we have created Project Management policies based on PMP® procedures as well as an integrated platform for internal and external process transfer named TT&GO®.
All our policies and platforms are provided to our customers with robust methodologies and procedures designed to minimize risks and maximize success as integral components of our overall Project Management strategy.
TT&GO

Suanfarma CDMO is highly committed to supporting its customers, which is why our teams focus on providing solutions to difficulties that may arise during the development of drug substance.
We have devised an innovative Technology Transfer Policy that, maximizes the chances of achieving our customers manufacturing objectives and minimizes risks during the early phases of the project.
Risk Based
Management
Systematic
methodology
To ensure fast and efficient transfer for development, scaling-up or commercialization.
QbD
(Quality by Design)
Gap and risk analysis
This innovative platform, registered under the name of TT&GO, follows a rigorous and systematic methodology based on quality criteria, enabling us to apply our GMP manufacturing knowledge in technology transfer processes. In addition, it ensures industrialization of any type of process in our plants and market commercialization of the final product quickly and efficiently.
Project management
Suanfarma CDMO's program management philosophy is to create and deliver innovative, robust and GMP compliant programs for our customers for a reliable and cost-effective project. Excellent communication, data sharing, and regular joint project reviews are key for the success of the program. At Suanfarma CDMO, we strive to grow with our customers, with their direct involvement.
Proven full scalability
We offer our CDMO customers alternatives and solutions adjusting adapted to the needs of each project. After years of experience in the industry, both in fermentation and chemical synthesis processes, as well as working on the development of our own molecules, our staff has the necessary understanding of the possible challenges that may arise both in the early stages of development of a molecule and in the scaling and commercialization phase of the manufacturing process.
Suanfarma CDMO has a proven track record in commercial development and manufacturing, having worked with more than 37 small molecules for pharma, biotech and healthcare CDMO customers worldwide and in highly regulated markets. The scalability of our technologies is proven, and our regulatory experts advise customers in the preparation of scaling strategies with the Medicines Agencies of the different regulated countries worldwide.
Regulatory and Quality
At Suanfarma CDMO we are guided by strict quality standards during all phases of production, from the selection of raw materials to manufacturing. Our facilities are in compliance with cGMP regulations required by EMA and FDA standards.
In our fermentation and chemical synthesis facilities, we have very demanding quality control in all stages, the development of analytical methods to the manufacturing process, from inspection of incoming materials to the release and continuous stability tests of finished products.
In addition, we offer our CDMO customers the experience of our quality assurance team, made up of professionals in charge of the management and preparation of registries and dossiers for the most demanding regulated markets in the world.






EGS
At Suanfarma we strive to guarantee the safety of our employees and the protection of the environment. Our environmental, social, and governance (ESG) agenda has become an essential part of our strategic development, including actions for full waste recycling, increasing green power and green chemistry, as well as proposing simpler and less environmentally harmful processes by combining bio fermentation, purification, and chemical synthesis in one site. Our strategies significantly improve environmental sustainability through innovative measures. We are clearly committed to having efficient manufacturing plants.
Expansion of our facilities
We are continuously expanding our facilities and staff. To meet the high demand for manufacturing capacity, we have invested in an expansion plan for one of our facilities, which will include three new DSP lines for purification in fermentative processes, allowing us to continue growing our CDMO service capacity, both in number of projects and requests.
Factory
I+D
Commercial office
Store
More information
For more information, please fill out the contact form. We will respond as soon as possible.