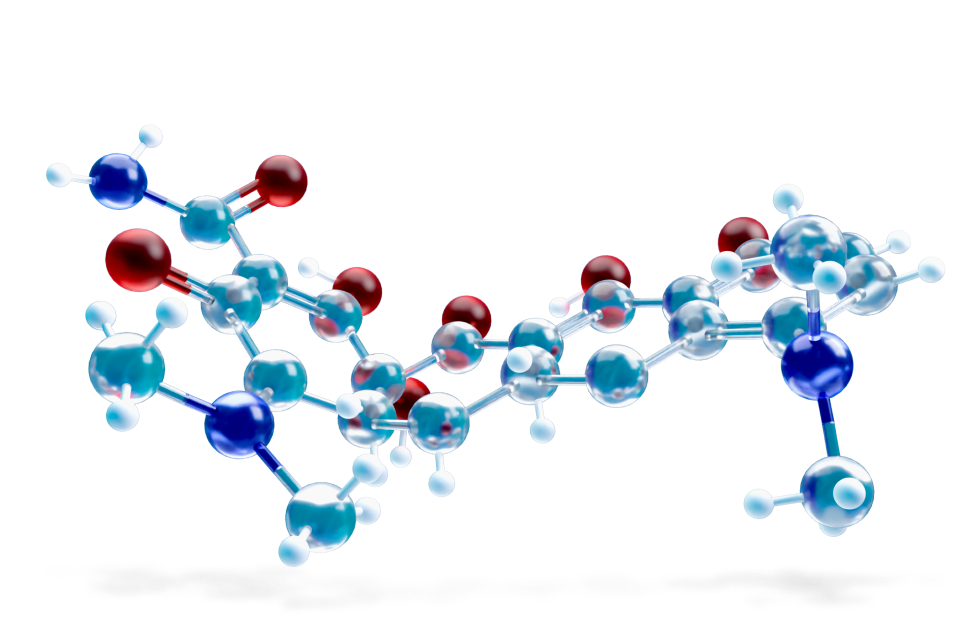
Also known simply as minicycline, is an antibiotic of the tetracycline group. It is bacteriostatic, classified as a long-acting type (it achieves plasma activities 2-4 times higher than its congeners). Minocycline is frequently used for the treatment of acne vulgaris and rosacea.
Much higher therapeutic adherence
Very wide antibiotic spectrum of action
Activity against Neisseria meningitidis.
Used to treat multidrug-resistant.
Better pharmacokinetic profile.
Longer half-life than Tetracycline.
FDA and European approval for the treatment of infections.
Low development of resistance in bacteria.
It is offered in diverse pharmaceutical forms, including oral administration (tablets or capsules), topical application, and injectable formulations:
Aerosol, Foam 1.5% and 4% Base
Injection 100 mg Base/Vial
Tablets:
50, 75 and 100 mg
Tablet, extended release:
45, 55, 65, 80, 90, 105, 115 and 135 mg
Hard capsules:
100 mg
USD global market sales
in 2022.
of the worldwide sales in
the US market
of the tetracycline
market
PATENT PROTECTION
CDMO
I+D
Calidad
RSC
Noticias
Contacto
Trabaja con nosotros